CAR-T Cell Therapy: Creating a New Era in Cancer Treatment
Cancer is one of the major health challenges facing society today. It can occur in various organs and tissues of the human body and has many types and subtypes. Traditional cancer treatment methods such as surgery, radiotherapy and chemotherapy can control the development of the disease to a certain extent, but at the same time, they also bring many side effects that cannot be ignored. Not only has it caused a great physical and psychological burden on the patient himself, but it has also had a profound impact on his family and society. With the rapid development of biotechnology, CAR-T cell therapy has attracted widespread attention as a new cancer treatment strategy.
1.What is Car-T cell therapy?
The concept of CAR-T cell therapy was first proposed in the 1980s. Researchers realized that by engineering a patient's T cells to recognize and attack cancer cells, a more powerful treatment could be achieved. With the development of genetic engineering technology, it is possible to construct chimeric antigen receptors (CAR). CAR is a protein composed of an antigen-binding domain and a signaling domain, which can recognize cancer cell-specific antigens on the surface of T cells and activate the killing function of T cells.
The first successful experiments with CAR-T cell therapy were conducted in the 1990s. Researchers introduced CAR into T cells and found that these modified cells can recognize and kill cancer cells, thus opening the door of research on CAR-T cell therapy. Over the ensuing decades, researchers have further refined and optimized CAR-T cell therapy.
CAR-T cell therapy made a remarkable breakthrough in the 2010s and achieved encouraging results in clinical trials. Especially in the treatment of acute lymphoblastic leukemia (ALL), CAR-T cell therapy has shown excellent efficacy. Many ALL patients have achieved long-term remission or even complete cure after receiving CAR-T cell therapy. This success has attracted widespread attention and promoted the research and application of CAR-T cell therapy in other cancer types.
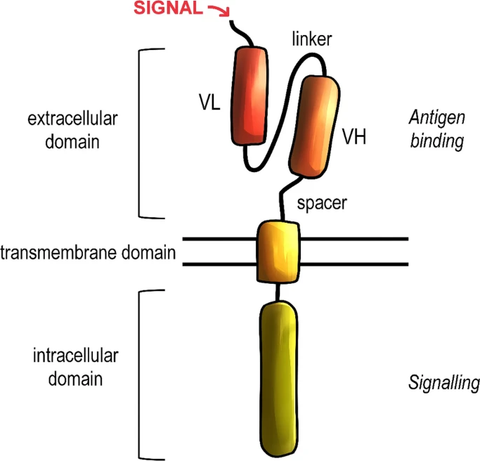
Fig.1 General structure of the chimeric antigen receptor (CAR). [1]
2.The principle of CAR-T cell therapy
CAR-T cell therapy is a genetic engineering technique that modifies a patient's own T cells to express a chimeric antigen receptor (CAR) with anti-tumor activity. CARs are usually composed of two main components: an external antigen-binding domain and an internal signaling domain. The antigen-binding domain recognizes specific antigens on the surface of cancer cells, while the signaling domain activates T cells and elicits an anti-tumor immune response. Once CAR-T cells are injected into a patient, they will actively seek out and destroy cancer cells expressing the target antigen, thereby playing a therapeutic role.
Car-T cell therapy usually includes the following five steps: 1. T cell collection: First, the doctor will collect T cells from the patient's blood, which is usually done through peripheral blood venous blood collection. The collected T cells are the patient's own immune cells, which reduces the risk of immune rejection. 2. CAR gene transduction: During cell processing, T cells are infected or transfected to introduce the CAR gene into their genome. The CAR gene encodes the CAR protein, which is a chimeric antigen receptor capable of recognizing and binding to cancer cell surface antigens. 3. CAR-T cell expansion: T cells transduced with CAR genes will continue to be cultured and expanded in the laboratory. This process is designed to increase the number of CAR-T cells to ensure that there are enough cells for treatment. 4. Treatment preparation: Once the CAR-T cells have passed quality control testing, they will be prepared for treatment. This may involve freezing and thawing the cells so that they are available to patients when they need them. 5. CAR-T cell infusion: After the CAR-T cells are prepared, they will be infused back into the patient's body. Typically, CAR-T cell infusions are given intravenously. Once infused, the CAR-T cells will begin to seek out and attack cancer cells.
After CAR-T cell therapy, patients will be closely monitored and managed. This may include monitoring the patient's response and side effects and providing supportive care and treatment. The goal of therapy monitoring and management is to ensure the best possible outcome for patients and to address any therapy-related issues in a timely manner.
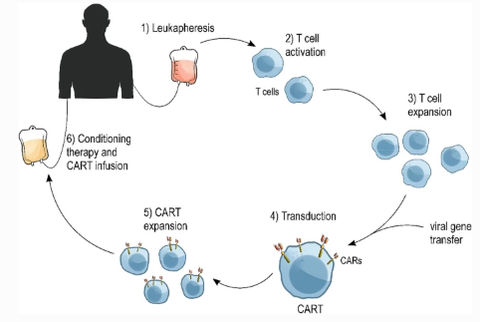
Fig.2 CART production and clinical use. [1]
3.Application Fields of CAR-T Cell Therapy
As an emerging immunotherapy, CAR-T cell therapy has shown significant application potential in the treatment of multiple cancer types.
The first was research on acute lymphoblastic leukemia (ALL), a subtype of leukemia that occurs primarily in immature cells of the lymphocyte lineage. It is the most common type of leukemia in children and adolescents, but it can also occur in adults. ALL is characterized by the abnormal proliferation and accumulation of malignant lymphoblastoid cells in the bone marrow, which are abnormal in the process of the development of mature cells from immature lymphocytes. These abnormal lymphocytes, called lymphoblasts or leukemia cells, replace normal blood-forming cells in the bone marrow and can invade other tissues and organs.
CAR-T cell therapy has shown significant application potential in the treatment of acute lymphoblastic leukemia (ALL) [2]. By introducing CAR into T cells, they can recognize and attack the CD19 antigen, a specific antigen on the surface of B lymphocytes that is widely expressed in most ALL patients. By introducing CD19-specific CAR into T cells, CAR-T cells can recognize and attack CD19 antigen-positive leukemia cells. This targeted therapy has a high degree of specificity and lethality, so it has achieved remarkable therapeutic effects in ALL patients. In addition, for refractory and relapsed ALL patients, traditional chemotherapy and stem cell transplantation have limited effects. CAR-T cell therapy offers a new treatment option for these patients. Clinical trials have shown that CAR-T cell therapy has achieved significant remission rates and long-term survival rates in refractory and relapsed ALL patients.
Secondly, CAR-T cell therapy has also achieved remarkable results in the treatment of relapsed or refractory non-Hodgkin's lymphoma [3]. Non-Hodgkin's lymphoma is a heterogeneous group of diseases, including multiple subtypes and subtypes, with different clinical features, molecular genetics, and treatment response. Non-Hodgkin's lymphoma is divided into various subtypes according to the cell origin and pathological features, such as diffuse large B-cell lymphoma (DLBCL), Burkitt Lymphoma (BL), follicular center lymphoma (FL) and so on.
Symptoms of non-Hodgkin lymphoma include swollen lymph nodes, weight loss, fatigue, night sweats, fever, itchy skin, and more. Diagnosis usually involves clinical examination, tissue biopsy, imaging studies, blood tests, and molecular genetic testing.
Diffuse large B-cell lymphoma is the most common subtype of non-Hodgkin lymphoma[4]. The application of CAR-T cell therapy targeting CD19 antigen has achieved remarkable therapeutic effects in patients with relapsed or refractory DLBCL. Clinical trials have shown that CD19 CAR-T cell therapy can induce a complete remission rate of up to 50% and significantly prolong the progression-free survival of patients. Tight B-cell lymphoma is a highly aggressive subtype of lymphoma. CAR-T cell therapy has shown potential efficacy in the treatment of tight B-cell lymphoma. CAR-T cell therapy targeting CD19 can achieve a high degree of therapeutic response and long-term remission. Follicle center lymphoma is a type of chronic non-Hodgkin lymphoma that is usually not curable. CAR-T cell therapy is also being investigated for the treatment of follicle center lymphoma. CAR-T cell therapy targeting CD19 antigen has shown some efficacy in patients with follicular center lymphoma[5].
The advantage of CAR-T cell therapy is that it can target specific antigens with high specificity and lethality. It has made a breakthrough in the treatment of non-Hodgkin's lymphoma, providing new treatment options for those refractory and relapsed patients.
4.Challenges and Prospects of CAR-T Cell Therapy
Due to the use of different cell sources and preparation processes, as well as differences in pretreatment and combination drugs, there are certain differences in the effects of various clinical projects, but overall, CAR-T cells are effective in treating tumors. The CAR-T products currently on the market are all second-generation CAR-T products. Now CAR-T technology has developed to the fifth generation:
The third-generation CAR-T technology adds a different co-stimulatory molecule to the second-generation CAR-T technology to improve proliferation and lethality.
The fourth-generation CAR-T has added suicide genes and can modify immune factors to prevent the side effects of CAR-T cytokine storm and finely regulate the human body.
The fifth-generation CAR-T technology will break through individual limitations, and can be used universally among different individuals for large-scale production and treatment.
Although the third, fourth, and fifth generations of CAR-T technology are still in the research and development stage, and the first and second generation CAR-T treatments are also quite expensive, but as Carl June said, "It's like when the computer just came out, a It would cost millions of dollars. But today, a home computer under $1,000 can do as much as it used to.” He believes that the advancement of technology will gradually lower the price, and eventually CAR-T technology will be able to fly into the homes of ordinary people. We have reason to believe that these technological innovations will gradually increase the stability and effectiveness of CAR-T, reduce its price and reduce side effects.
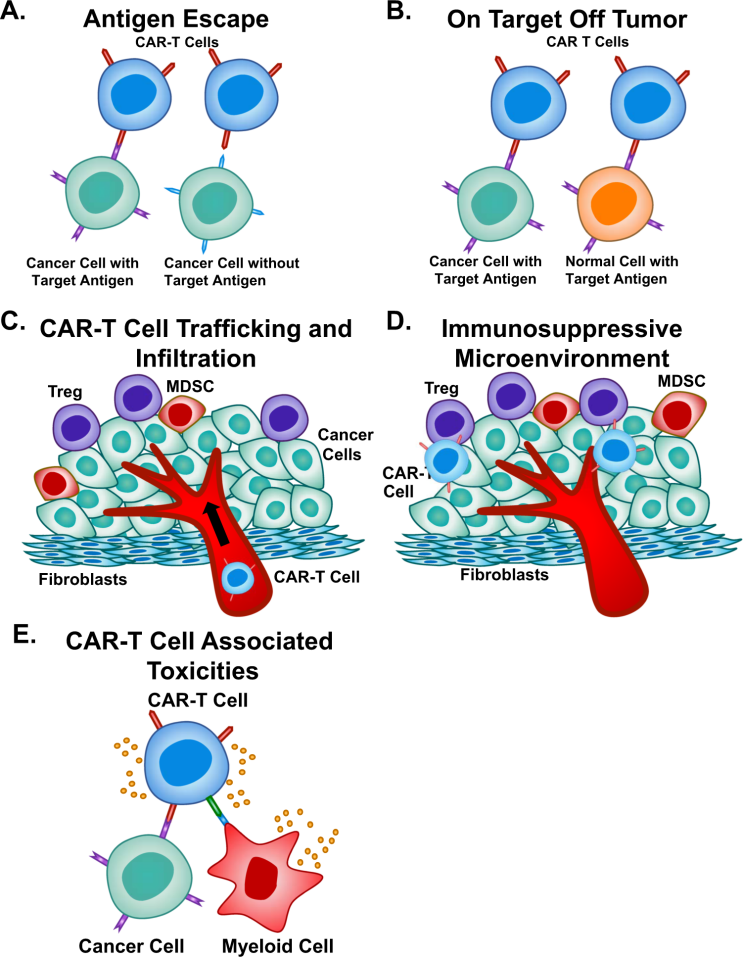
Fig. 3: Limitations of CAR-T Cell Therapy. [6]
5.Future Prospects for Car-t Cell Therapy
Looking ahead, CAR-T cell therapy will continue to be an important research direction in the field of cancer treatment. By improving the CAR structure and adjusting the preparation process of CAR-T cells, the therapeutic effect can be further improved and the side effects can be reduced. Furthermore, combining other immunotherapeutic approaches, such as immune checkpoint inhibitors and vaccines, may yield better therapeutic outcomes. With the advancement of science and technology, CAR-T cell therapy is expected to play a greater role in cancer treatment and bring more hope to patients.
As an innovative cancer treatment strategy, CAR-T cell therapy provides us with a new way to fight this severe disease. Despite some challenges, its unique mechanism and remarkable clinical effects demonstrate its great potential. With the continuous improvement of technology and the advancement of clinical practice, CAR-T cell therapy will bring more hope to cancer patients and open up a new era for cancer treatment.
| generation | CAR-T factor modification | Effect |
|---|---|---|
| first generation | CD3ζ | Activated T cells in vitro, with conventional T cell killing toxicity, but unable to proliferate and survive for a long time in vivo |
| second generation | CD3ζ+CD28/CD137 | Adding a co-stimulatory molecule prolongs the survival time in the body, improves the proliferation ability and antivirus ability |
| Third Generation | CD3+CD28/CD134/CD137+CD28/CD134/CD137 | Adding two different co-stimulatory molecules, the proliferation ability and killing ability are further improved |
| Fourth Generation | Suicide gene/CAR-T(IL-12)/PD-1 | Added various integrated and fine-tuned regulation methods such as suicide genes and immune factor modification |
| Fifth Generation | Universal CAR-T, gene editing | No individual limitation, large-scale production and treatment |
References:
[1] Skorka, K., Ostapinska, K., Malesa, A. et al. The Application of CAR-T Cells in Haematological Malignancies. Arch. Immunol. Ther. Exp. 68, 34 (2020). https://doi.org/10.1007/s00005-020-00599-x
[2] Valle, P. A. , Coria, L. N. , Plata, C. , & Salazar, Y. . (2021). Car-t cell therapy for the treatment of all: eradication conditions and in silico experimentation.
[3] Fuerst, M. L. . (2018). Car t-cell therapy induces long-lasting responses in refractory nhl. Oncology Times, 40.
[4] Riedell, P. A. . (2020). Advances in car t-cell therapy for aggressive b-nhl. Clinical Lymphoma, Myeloma and Leukemia, 20, S94-S97.
[5] Xiao, Z. , & Jingting, J. . (2018). Current situation and clinical application prospect of CAR-T cell therapy.
[6] Sterner, R.C., Sterner, R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021). https://doi.org/10.1038/s41408-021-00459-7
[7] Williams, M. E. . (2014). Further Progress in CAR-T-Cell Therapy for Acute Lymphoblastic Leukemia.
[8] Neubauer, A. . (2019). Car t-cell therapy. Arzneimitteltherapie, 37(3), 69-72.
[9] Preparing for car t cell therapy: patient selection, bridging therapies and lymphodepletion. Nature Reviews Clinical Oncology.












