Recombinant Growth Factors for Organoid Culture - Wnt3a
Recently, researchers from the Cincinnati Children's Hospital Medical Center in the United States published a study in the journal Nature Biotechnology, introducing a new tool for studying gastrointestinal immunity. They successfully transplanted human intestinal organoids (HIOs) derived from pluripotent stem cells into the renal capsules of mice with humanized immune systems. This led to the establishment of next-generation HIOs in vivo models with functional human immune tissues. The lack of an immune system has always been a limitation in organoid systems, and this study provides a new approach for establishing immune-competent organoid models.
Organoids and Wnt Signaling Pathway
Organoids are three-dimensional structures established from pluripotent stem cells (PSCs) or adult tissue stem cells (ASCs). In vitro organoid culture systems consist of a population of self-renewing stem cells that can differentiate into multiple organ-specific cell types, mimicking the spatial organization and partially reproducing the functions of the corresponding organs.
From the definition of organoids, it is clear that the generation of in vitro organoids relies on the growth, development, and maturation of stem cells. The classical Wnt signaling pathway, specifically the Wnt/β-catenin pathway, regulates the pluripotent differentiation of stem cells, organ development, and regeneration. Wnt signaling is an important pathway for stem cells and is involved in the differentiation of various cell types, including cardiomyocytes and intestinal epithelial cells.
How Wnts Support Organoid Culture
1. Gastrointestinal Organoids
In 2009, it was a revolutionary year in the field of organoids. The pioneer in organoid research, Hans Clevers, and his team, including Sato, successfully isolated individual Lgr5+ stem cells from the crypts of the mouse small intestine. In a culture environment with extracellular matrix (3D support), the stem cells were cultivated by adding cell factors such as epidermal growth factor (EGF), bone morphogenetic protein antagonist (Noggin), Wnts, and R-Spondin1 (Wnt signaling activation). As a result, the formation of intestinal villi and crypt-like structures was observed, successfully establishing an in vitro intestinal organoid. These organoids are hollow spheres that contain complete intestinal epithelial structures and all types of functional intestinal epithelial cells, including intestinal epithelial cells, enteroendocrine cells, goblet cells, Paneth cells, and Lgr5+ stem cells [1].
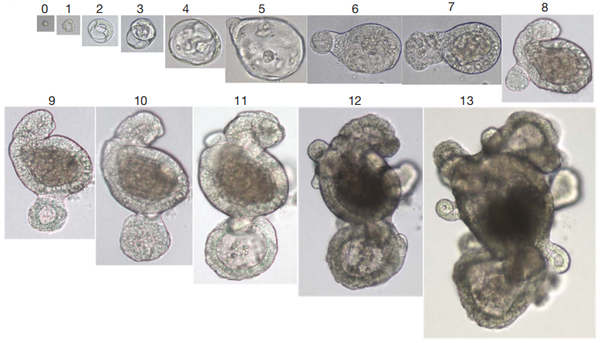
2. Cardiac Organoids
Hofbauer et al. [2] successfully developed cardiac organoids derived from human induced pluripotent stem cells (hiPSCs). By introducing key signaling pathway factors involved in heart development (Activin, BMP, FGF, retinoic acid, and Wnt), the hiPSC-derived cardiac organoids underwent morphogenesis and morphological changes to form a cavity. These organoids differentiated into distinct layers of cardiomyocytes and endothelial cells and interacted with migrating and differentiating epicardial cells, thus mimicking early cardiac chamber development. Self-organizing cardiac organoids can demonstrate the processes of morphogenesis and cell differentiation in regions such as the myocardium, endothelium, and epicardium, providing a powerful system for studying the mechanisms of human cardiac development and congenital heart disease.
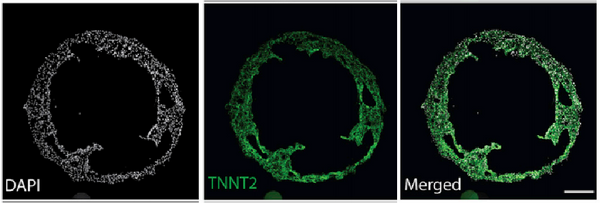
and the expression of a cardiomyocyte-specific marker, TNNT2 [2].
3. Other Organoids
In addition to gastrointestinal and cardiac organoids, Wnts have also been instrumental in the successful establishment of many other types of organoids, including gastric organoids [3] and liver organoids [4].
Wnt3a Protein
The Wnt family is a highly conserved, cysteine-rich, large secreted protein family that is important for normal developmental processes. Two types of Wnt secreted proteins have been identified. One type is the classical Wnt signaling pathway that depends on β-catenin, and Wnt 3a is the major ligand of the classical Wnt signaling pathway. Wnt3a signals through the Frizzled (FZD) receptor family and the LRP5/6 co-receptor to regulate gene expression and various cellular behaviors [5].
BetaLifeScience provides high purity, high activity, and low endotoxin Wnt3a V3 protein (Cat# BL-2913NP), which has been validated in organoid platforms for its ability to promote organoid growth.
Product features:
(1) High purity and high activity
(2) Low endotoxin levels (<10 EU/mg, below the industry average)
(3) High batch-to-batch consistency
(4) Free of carrier proteins
(5) Available in stock
(6) Various packaging options available: 10μg, 50μg, 500μg, 1mg.
Sample Product Data
1. Product Activity
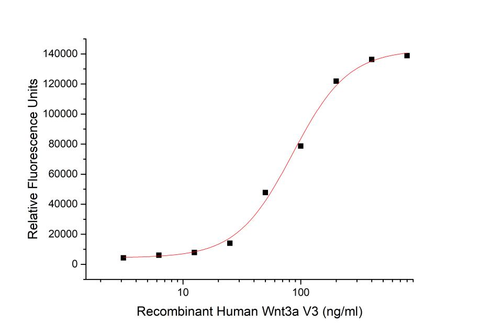
The ED50 for this effect is 71.45 ng/ml.
2. Establishment of a Mouse Gastrointestinal Organoid Culture System
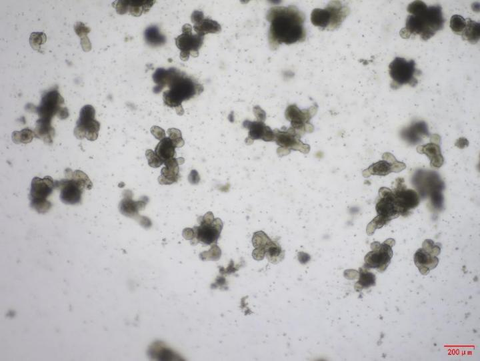
Noggin (Cat#BL-0001NP) and RSPO1 (Cat#BL-0319NP).
3. Establishment of a Mouse Gastric Organoid Culture System
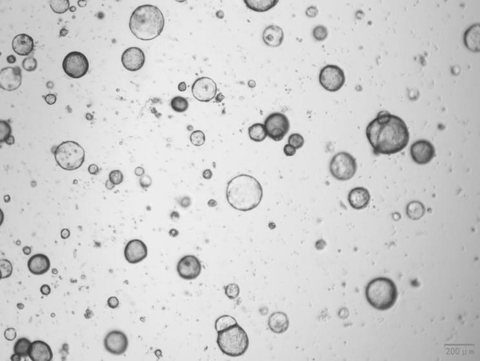
Noggin (Cat#BL-0001NP) , RSPO1 (Cat#BL-0319NP) and FGF-10 (Cat#BL-1955NP).
The organoids showed good morphology.
4. The following factors have been validated and successfully used in establishing a human pituitary tumor organoid culture system:
- IGF1 (#BL-1689NP)
- Wnt3a (#BL-2913NP)
- RSPO1 (#BL-0319NP)
- FGFb (#BL-2872NP)
- FGF8b (#BL-2874NP)
- FGF10 (#BL-1955NP)
- SHH (#BL-2024NP)
- Noggin (#BL-0001NP)
Recommended Low Endotoxin Cytokines for Organoid Culture
References
[1] Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009 May 14;459(7244):262-5.
[2] Hofbauer P, et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell. 2021 Jun 10;184(12):3299-3317.e22.
[3] Bartfeld S,et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015 Jan;148(1):126-136.e6.
[4] Jeong J, et al. Elimination of Reprogramming Transgenes Facilitates the Differentiation of Induced Pluripotent Stem Cells into Hepatocyte-like Cells and Hepatic Organoids. Biology (Basel). 2022 Mar 23;11(4):493.
[5] Nusse R et al. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell (2017).












