The Role of PD-1 in Immune Checkpoint Regulation
Programmed death protein 1 (PD1) is a common immunosuppressive member on the surface of T cells and plays an imperative part in downregulating the immune system and advancing self-tolerance. Its ligand programmed cell death ligand 1 (PDL1) is overexpressed on the surface of malignant tumor cells, where it binds to PD1, inhibits the proliferation of PD1-positive cells, and participates in the immune evasion of tumors leading to treatment failure. The PD1/PDL1-based pathway is of great value in immunotherapy of cancer and has become an important immune checkpoint in recent years.
The Structure and Function of PD-1
PD-1 is a type I membrane protein composed of 288 amino acids. As a member of the CD28/CTLA-4 family of T cell regulators, PD-1 plays a crucial role in modulating T cell responses. The protein's structure consists of an extracellular IgV domain, a transmembrane region, and an intracellular tail[2]. Within the intracellular tail, two phosphorylation sites are present, forming an immunoreceptor tyrosine-based inhibitory motif and an immunoreceptor tyrosine-based switch motif. These elements indicate that PD-1 acts as a negative regulator of T-cell receptor (TCR) signaling.
Upon ligand binding, PD-1's cytoplasmic tail interacts with SHP-1 and SHP-2 phosphatases, further enhancing its inhibitory function[3]. Additionally, PD-1 binding upregulates the E3-ubiquitin ligases CBL-b and c-CBL, leading to the downregulation of T cell receptors, dampening T cell activation, and reducing cytokine release. PD-1 is expressed on the surface of activated T cells, B cells, and macrophages, suggesting its broader role in negatively regulating immune responses compared to CTLA-4.
Expression and Regulation of PD-1
PD-1 is prominently expressed in activated CD4+ and CD8+ T cells, activated B cells, natural killer (NK) cells, natural killer T cells, dendritic cells (DC), and activated monocytes, and its expression is closely linked to their differentiation and apoptosis.
The expression of PD-1 can be induced by activation of T cell receptors (TCR) or B cell receptors, and tumor necrosis factor can further enhance PD-1 expression.
The wide-ranging expression of PD-1 highlights its crucial role in maintaining a negative immune response within the body.
The Signaling Pathway of PD-1/PD-L1
Under normal conditions, the body's immune system has an immune surveillance function. When malignant cells appear, the immune system can specifically recognize and remove these “nonself” cells to prevent tumor growth. However, in some cases, malignant cells prohibit immune responses against tumors by upregulating immunosuppressive molecules or downregulating immune-activated molecules, thereby achieving immune escape and immortalization[5]. PD-1/PD-L1 has been the most studied negative regulatory immune checkpoint-related axis in recent years and plays a prominent role in tumor immune escape.PD-L1 (CD274) and PD-L2 (CD273) are two ligands of PD-1 consisting of 290 and 270 amino acid residues, respectively, which are also type I transmembrane proteins and belong to the B7/CD28 family, and PD-L1 and PD-L2 have 37% sequence homology. PD-L1 has a wider range of expression than PD-L2, resulting in PD-L1 playing a major part in tumor cell immune escape [7-8].
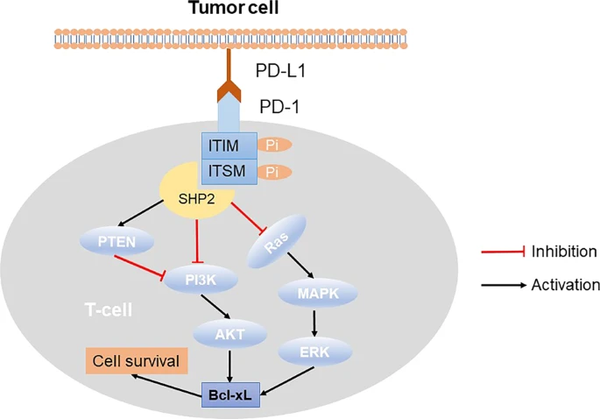
The structure of PD-1 consists of four parts: an immunoglobulin variable region (IgV), a transmembrane region, immunoreceptor tyrosine-based inhibitory motifs (ITIMs), and immunoreceptor tyrosine-based switch motifs (ITSMs) [6, 9]. The interaction of PD-1 and PD-L1 induces ITIMs and ITSMs to be phosphorylated in the intracellular domain of PD-1, which recruits tyrosine acid phosphatase Src homology phosphatase 1 (SHP-1) and Src homology phosphatase 2 (SHP-2) [9-10]. These phosphatases dephosphorylates several key proteins in the T cell antigen receptor (TCR) signaling pathway and repress signaling pathways downstream of the TCR, such as phosphoinositide 3-kinase (PI3K), protein kinase B (PKB/AKT), mammalian target of rapamycin (mTOR), rat sarcoma (RAS), mitogen-activated protein kinase (MAPK/MEK), extracellular regulated protein kinase (ERK), etc. In turn, it inhibits the transcription of related genes, hinders the progression of T cell cycle and the expression of related proteins, and ultimately blunts the production of cytokines and the proliferation and differentiation of T cells, causing their immune function to be lost [11-13].
PD-1 and Cancer Immunotherapy
PD-1 plays a critical role in cancer immunotherapy and has become a prominent target in current tumor treatment approaches. Cancer cells have the ability to evade the immune system by exploiting immune checkpoint signaling pathways, leading to the suppression of the immune response against tumors. PD-1 is a crucial protein in the immune checkpoint, playing a vital role in regulating T cell activation and immune tolerance.
In the tumor microenvironment, both tumor cells and immune cells express PD-L1, which serves as the ligand for PD-1. When PD-L1 binds to PD-1, it triggers an inhibitory signal, dampening the activation and attacking capabilities of T cells. As a result, tumor cells remain protected from immune system attacks, contributing to tumor growth and metastasis.
To counteract this immune evasion mechanism, researchers have developed PD-1 inhibitors, which effectively block the binding of PD-1 and PD-L1. By doing so, these inhibitors restore T cell activation and enhance the immune cells' ability to attack tumor cells. This anti-PD-1/PD-L1 immunotherapy has demonstrated remarkable clinical results and proven significant efficacy in treating various malignant tumors. It not only leads to tumor shrinkage and control but also improves patient survival and quality of life.
PD-1 and Autoimmune Diseases
PD-1 plays a significant role in autoimmune diseases due to its function as an essential immune checkpoint protein. Its primary role is to inhibit the activation of immune cells, particularly T cells, by binding to its ligands PD-L1 and PD-L2. This regulation prevents excessive immune responses and attacks on the body's own tissues. However, when the PD-1 signaling pathway becomes dysregulated, it can lead to the immune system attacking self-tissues, resulting in autoimmune diseases.
Autoimmune diseases are characterized by the immune system mistakenly attacking the body's own tissues or cells. Some common examples include rheumatoid arthritis, systemic lupus erythematosus, thyroid autoimmune disease, and inflammatory bowel disease.
In autoimmune diseases, abnormal expression or dysfunction of PD-1 and its ligands PD-L1 and PD-L2 can cause excessive suppression of immune cells, disrupting the balance and regulation of the immune system. This imbalance leads to autoimmune attacks, where immune cells may attack normal tissues, causing damage to organs and tissues.
Therapeutic approaches targeting the PD-1/PD-L1 signaling pathway have become crucial in treating autoimmune diseases. PD-1 inhibitors can restore the activation of immune cells and block autoimmune attacks, thereby reducing disease symptoms and improving patients' quality of life. However, it is important to note that the use of PD-1 inhibitors in autoimmune disease treatment may also cause immune-related side effects. Therefore, close monitoring and timely treatment are essential to manage potential adverse effects.
The Clinical Significance of PD-1
The clinical significance of PD-1 (programmed death protein 1) is particularly important in the field of cancer immunotherapy. PD-1 is a member of the immune checkpoint, and its main function is to regulate the activation state of T cells to maintain the balance of the immune system and avoid excessive immune responses. However, in some malignant tumors, tumor cells will use the PD-1 signaling pathway to bind to its ligand PD-L1 to inhibit the function of T cells, thereby avoiding being attacked by the immune system and achieving immune escape, resulting in the inability of the tumor to be detected. Effective removal.
The clinical significance of PD-1 is that immune checkpoint inhibitors targeting the PD-1/PD-L1 signaling pathway have become a revolutionary anti-cancer treatment strategy. These inhibitors can block the binding of PD-1 to PD-L1 and restore the function of T cells so that they can attack tumor cells. This treatment method has achieved remarkable clinical efficacy in various cancer types, especially in the treatment of malignant tumors such as non-small cell lung cancer, melanoma, and renal cell carcinoma.
The clinical application of PD-1 inhibitors not only significantly improves the survival rate of patients, but also provides new treatment options for those patients with advanced or refractory cancer. Many patients have experienced long-term tumor remission after receiving PD-1 inhibitor therapy, which is a major breakthrough for patients who have been unable to effectively control their tumors.
PD-1 Protein
Recombinant Human Programmed cell death 1 ligand 1(CD274) Protein (hFc), Active
High Purity Validated by SDS-PAGE
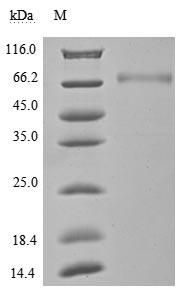
The purity was greater than 95% as determined by SDS-PAGE.(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
Excellent Bioactivity Validated by Functional ELISA
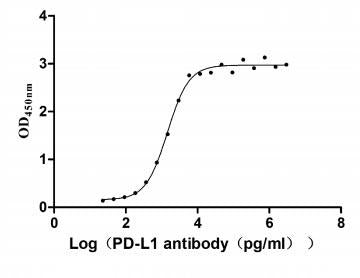
Measured by its binding ability in a functional ELISA. Immobilized PD-L1 at 2 μg/ml can bind Anti- PD-L1 mouse monoclonal antibody (antigen from E.coli), the EC50 of human PD-L1 protein is 1.252-1.653 ng/mL.
References:
[1] Zak KM, Grudnik P, Magiera K, Dömling A, Dubin G, Holak TA. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Structure. 2017;25(8):1163-1174. doi:10.1016/j.str.2017.06.011
[2] Kinter, A. L., Godbout, E. J., McNally, J. P., Sereti, I., Roby, G. A., O'Shea, M. A., et al. (2008). The Common Gamma-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and its Ligands. J. Immunol. 181 (10), 6738–6746.
[3] Kulpa, D. A., Lawani, M., Cooper, A., Peretz, Y., Ahlers, J., and Sékaly, R. P. (2013). PD-1 Coinhibitory Signals: the Link between Pathogenesis and protection. Semin. Immunol. 25 (3), 219–227. doi:10.1016/j.smim.2013.02.002
[4] Wu, Q., Jiang, L., Li, Sc. et al. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol Sin 42, 1–9 (2021). https://doi.org/10.1038/s41401-020-0366-x
[5] Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450-461. doi:10.1016/j.ccell.2015.03.001
[6] Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016;375(18):1767-1778. doi:10.1056/NEJMra1514296
[7] Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336-347. doi:10.1038/nri1349
[8] Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94(1):25-39. doi:10.1189/jlb.1212621
[9] Lin DY, Tanaka Y, Iwasaki M, et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105(8):3011-3016. doi:10.1073/pnas.0712278105
[10] Lázár-Molnár E, Yan Q, Cao E, Ramagopal U, Nathenson SG, Almo SC. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A. 2008;105(30):10483-10488. doi:10.1073/pnas.0804453105
[11] Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153-167. doi:10.1038/nri.2017.108
[12] Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428-1433. doi:10.1126/science.aaf1292
[13] Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. Published 2012 Jun 26. doi:10.1126/scisignal.2002796





